
A fluorochrome is excited, and emits a photon in a range of wavelengths.
COMPENSATION FLOWJO SOFTWARE
If you run a flow cytometer that does not have an automatic compensation package, it is best to collect your data uncompensated and use a third party software package to perform compensation.
COMPENSATION FLOWJO MANUAL
There is no reason an investigator should be performing manual compensation with the propagation of different tools for the job. As described in that paper, and shown in Figure 5 (Figure 2 from Bagwell and Adams’ paper), there are several ways to do this, but the most accurate method is to use automatic compensation ( Herzenberg et al. It is derived from a paper by Bagwell and Adams (1993) (Ann NY Acad Sci 20:167-184). The more accurate and gold standard for compensation is automatic compensation. If you are using an instrument and still performing manual compensation, please ensure you are using statistical data to generate this value. The slope of the line based on the median measurements of the positive and negative gates is calculated to determine the compensation value. This is the spillover of fluorescein into the phycoerythrin (PE) channel.įigure 4: Compensation of the Fluorescein in the PE detector. However, as you can see, there is a percentage of this signal also in the 585/42 filter (approximately 12%). In Figure 2, below, the fluorescein signal is being measured in the BandPass 530/30 filter. PMTs will generate a photocurrent if any photon strikes them therefore, we use filters to restrict the light going to each PMT. As we’ve discussed previously flow cytometers use photomultiplier tubes (PMT) to convert photons to electrons. The problem comes in when we wish to measure this fluorescein signal. As shown in the Figure 1 for fluorescein, this emission occurs at a maximum of about 519 nm, but stretches out far into the orange spectrum (over 600 nm). To release that energy, the molecule emits a photon of a higher wavelength. In the process of excitation, the fluorescent molecule is excited that is, an electron is moved to a higher energy state. It all starts with fluorescence that is, the emission of a photon of light from a molecule that has absorbed a photon of light of a lower wavelength. Figure generated using the Invitrogen spectral viewer. The maximal emission is ~519 nm however fluorescein can emit a photon out into the yellow and red regions of the spectrum. The emission profile, shown in the filled green, represents the probability a photon of a given wavelength will be emitted. The dashed line shows the wavelengths of light that can excite the fluorescein molecule, up to the maximal excitation of ~490 nm.
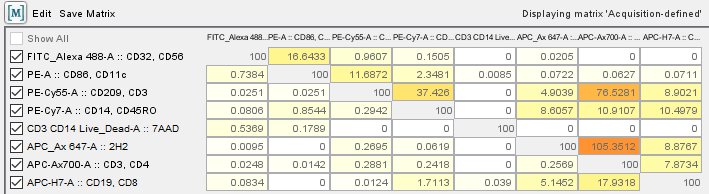
Figure 1: Fluorescein excitation and emission spectrum.


 0 kommentar(er)
0 kommentar(er)
